Monocytosis Workflow Optimisation
Support to distinguish reactive and malignant monocytosis
- Reduce unnecessary smear reviews for reactive monocytosis and focus on true positive samples
- Proven balance between clinical insight (sensitivity) and workflow (specificity)
- Save TAT and costs
- Detect signs suggestive of dysplasia from your routine CBC+DIFF analysis
- Add-on that incorporates some research use-only parameters
- Add-on that requires validation by the user before implementing it in routine clinical work
Where do you set your MONO# cut-off for smears? Balancing the burden of the smear workload with the risk of missing critical samples is not easy. The Monocytosis Workflow Optimisation (MWO) offers an evidence-based* optional rule set embedded in the Extended IPU to distinguish reactive monocytosis from monocytosis of suspected malignant origin.
Monocytosis management – The dilemma with selecting the optimum criteria for morphological review
Existing criteria for monocytosis management differ in the MONO cut-off criteria - ISLH/GFHC suggest a smear review for MONO# > 1.5 x103/µL, while according to the WHO (2022), chronic myelomonocytic leukaemia (CMML), a rare leukaemia, is characterised by a MONO# ≥ 0.5 x 103/μL and ≥ 10% of the WBC count.
Reactive cases are most commonly the cause of monocytosis. However, there is a small possibility that the monocytosis is of malignant origin. This suspicion leads to microscopic examination, generating a high number of unnecessary smears. This leads to the dilemma of finding a balance between a significant increase of smears on false-positive samples to not miss a single case of suspected CMML and the reduction of the manual workload with the inherent risk of missing a rare abnormality due to a low sensitivity for CMML detection.
The MWO concept - How does it work?
MWO combines the so-called ‘mono-dysplasia score’, the monocyte counts and information from the WDF scattergram to recommend samples with monocytosis for microscopic examination.
The ‘mono-dysplasia score’, established and assessed by Schillinger et al., is calculated from three analytical components obtained from the WDF measurement in the search for dysplastic abnormalities typical for CMML.
Parameters
- Mono #
- Neut #/Mono #
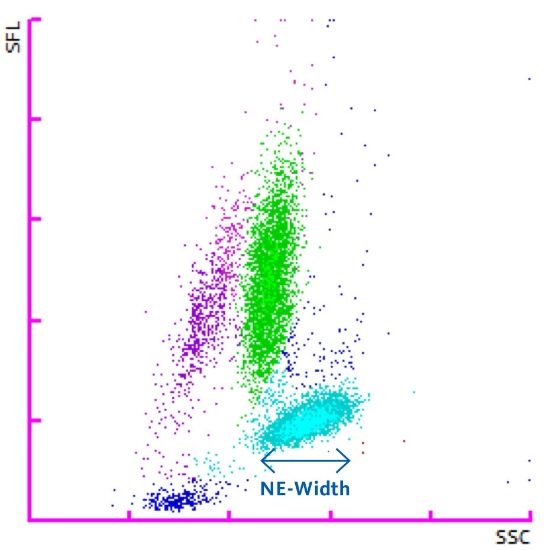
- NE-WX
Scatterplot showing the dispersion of the neutrophil population from which the NE-WX is obtained
References
*Schillinger F et al. (2018): A new approach for diagnosing chronic myelomonocytic leukemia using structural parameters of Sysmex XNTM analyzers in routine laboratory practice. Scand J Clin Lab Invest. Vol. 78(3):159–164.
Sysmex Norway NUF
Hvamsvingen 24
2013 Skjetten
Norway
+47 63840160
Product documents
Regulatory Documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex