Sysmex‘ integrative solutions for prostate cancer
In Europe, more than 600,000 men are diagnosed with a urological malignancy each year,1 with prostate cancer being the second most common cancer in men. If found early, prostate cancer is highly treatable and, in some cases, surgery alone can be curative.2-3 Our solutions are designed to help the laboratories, clinicians, and patients that address screening, surgery, diagnosis and therapy of prostate cancer.
A rapid and accurate PSA Test supports early detection and monitoring of prostate cancer at the point of care.
Early detection is an important factor in getting appropriate and timely treatment. Thanks to screening programs, prostate cancer mortality has significantly reduced in recent years across Europe.
A quantitative PSA test is particularly important in combination with other diagnostic methods in the clarification of suspected cancer. Therefore, a typical prostate cancer screening consists of a combination of at least two tests: the Digital Rectal Examination (DRE) and the PSA test.
The PSA test is a blood test that measures the presence of prostate-specific antigen (PSA), a protein produced by both cancerous and non-cancerous cells in the prostate and is in the hands of an experienced physician an important tool.
It is used not only for effective screening for prostate cancer but also for monitoring the disease and the success of therapy.
Please visit https://www.hitado.de/psa in German or https://www.eurolyser.com/medical-diagnostics/parameter/psa-test/ in English for more information.
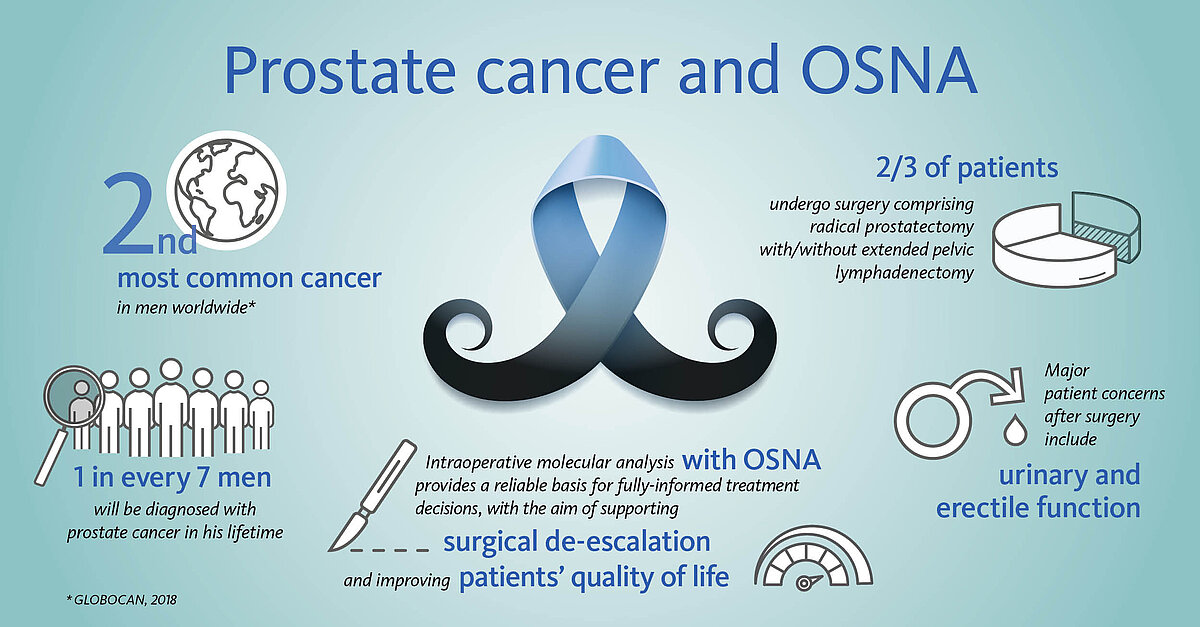
Accurate intraoperative lymph node analysis with OSNA supports de-escalation of surgical radicality
Most men undergoing surgery will have not only their prostate removed, but also the pelvic lymph nodes (LN) in order to eliminate any potential source of tumour cells. While the removal of the prostate is associated with risks of urinary and erectile dysfunctions, the removal of the adjacent LNs increases the chances of lymphatic drainage dysfunctions.
However, in some 70-90% of early stage patients the tumour is confined to the prostate, making the extra burden of the node dissection curatively pointless.
Could extensive LN dissection and related morbidity be spared in these men?
Pioneer groups in the field of prostate cancer advocate innovative, less invasive treatment approaches by reducing the number of LNs that are removed to a bare minimum. Namely the sentinel lymph node biopsy, a well-stablished method in several other malignancies; here, only the node(s) most prone to carry metastasis are removed and analysed4-6.
The OSNA molecular diagnostic test has been successfully supporting the accurate analysis of sentinel LNs in breast cancer patients worldwide for over 10 years. As of 2020, OSNA technology is also available for prostate cancer patients. OSNA provides fast and accurate results intraoperatively, thus supporting de-escalation of surgery and potentially improving prostate cancer patients’ quality of life.

On the way to personalised medicine with SureSeq myPanel™ NGS Prostate Cancer panel
While many genes can become mutated during the onset and development of prostate cancer, some mutations may be inherited.1 Patients identified with certain genetic mutations can benefit from increased monitoring, preventative steps and/or targeted therapy.
Nowadays, a range of anti-tumour drugs targeting specific genetic mutations are available, and can be adjuvant to standard chemo/immuno/hormonal and radiation therapies. To investigate such mutations, oncologists or urologists may request a genetic test by next generation sequencing (NGS) which may support them in making personalised treatment decisions.
SureSeq myPanelTM NGS Prostate Cancer panel offers customised NGS tests that interrogate critical and currently relevant prostate cancer genes including: ATM, BRCA1, BRCA2 and PALB2. In combination with OGT’s complimentary Interpret NGS analysis software, this ensures an effortless translation of NGS data into meaningful results.
For further information go to: SureSeq myPanel™ NGS Custom Prostate Cancer Panel website
Disclaimer
*SureSeq: For research use only; not for use in diagnostic procedures.
Literature
- Ferlay J et al.(2013): Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 49(6):1374-403.
- Bekelman JE et al. (2018): Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol.:JCO1800606.
- Mottet N et al. (2017): EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 71(4):618-629.
- van der Poel HG et al.(2017): Sentinel Node Prostate Cancer Consensus Panel Group members. Sentinel node biopsy for prostate cancer: report from a consensus panel meeting. BJU Int. 120(2):204-211.
- van der Poel HG et al. (2017): Sentinel node biopsy and lymphatic mapping in penile and prostate cancer. Urologe A. 56(1):13-17.
- Wit EMK et al. (2017): Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy. Eur Urol. 71(4):596-605.
- Thoma, C, (2015) The complex relationships of malignant cells in lethal metastatic castration-resistant disease, Nature Reviews Urology 12, 237